Continuous control and management of mycotoxins involve a series of crucial steps. The process starts off at sampling, which is a key step in the analysis. Subsequently, grinding and homogenizing of the sample are performed, followed by reading of the spectrum in the infrared equipment and, lastly, interpretation of the result.
Such complex set of actions aids in establishing the Mycotoxins Risk. Furthermore, data interpretation determines the decision-making regarding the choice of including an Anti-mycotoxins Additive the diet.
Steps of mycotoxins management
1 - Sampling
Pegasus Science evaluates which sampling plan suits the reality of the Company best. Afterwards, Pegasus Science provides sampling training for the technical capacitation of the team.
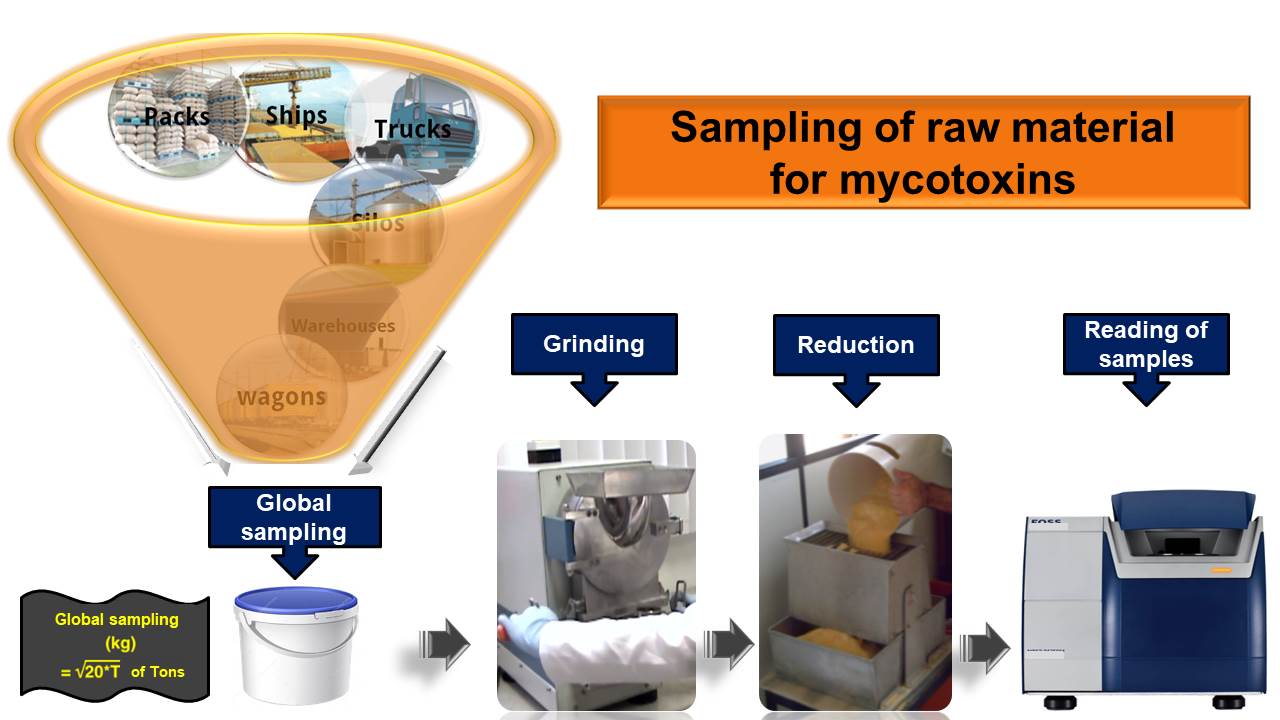
2 - Mycotoxins analysis
Sample preparation consists of grinding and homogenizing, which are carried out in the Company’s production plant. An aliquot of the sample is then placed in the reading cell of the NIR equipment for reading of the spectrum. Next, the file with the spectrum will be sent to Pegasus Science via Olimpo Platform. The results of the analyses will be automatically provided.
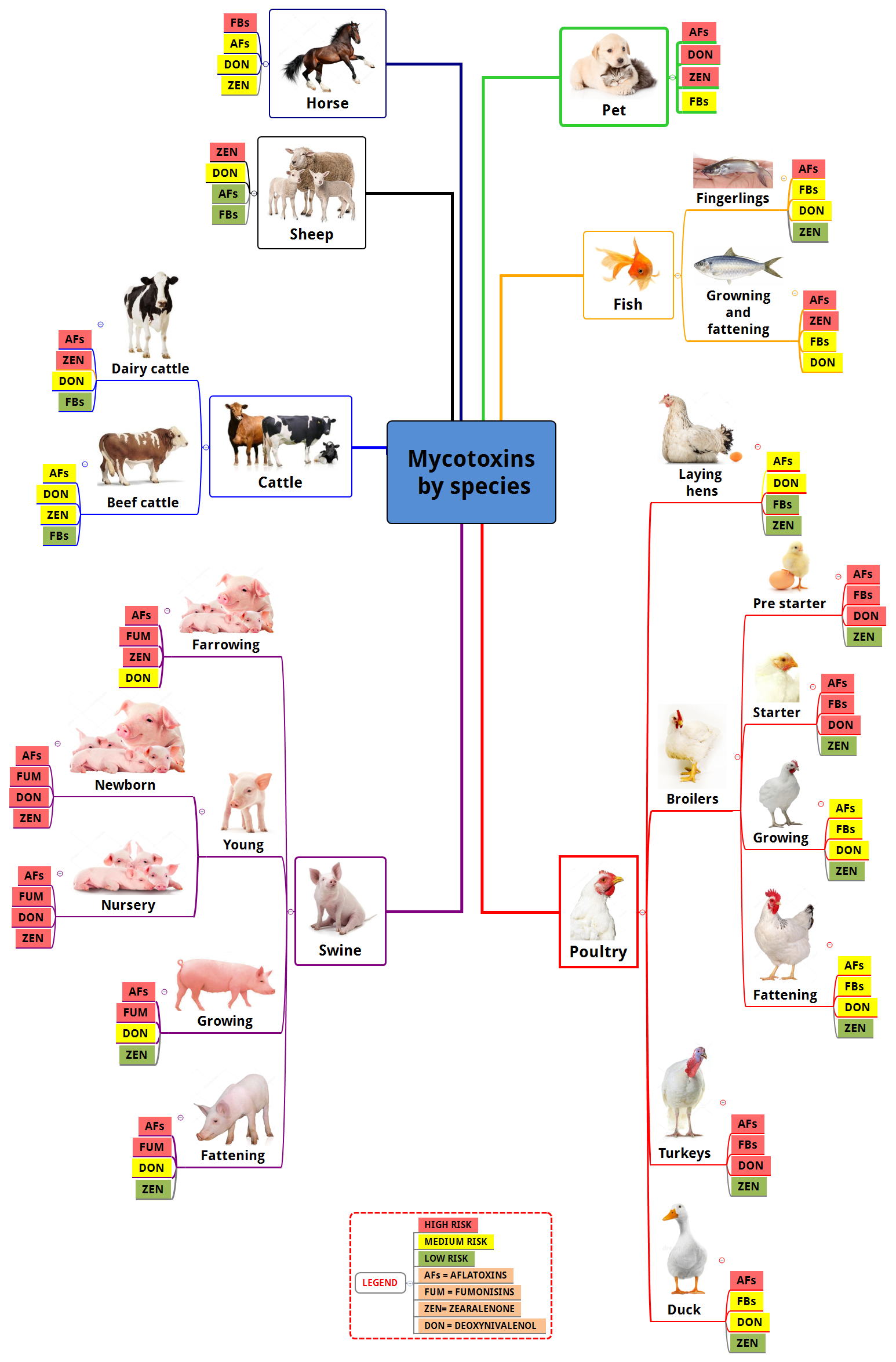
Which mycotoxins should be analyzed? Those which have the greatest prevalence in a given raw material, also considering the susceptibility of the animal species and the production phase to which the ingredient will be offered.
Susceptibility of a diversity of animal species to mycotoxins:
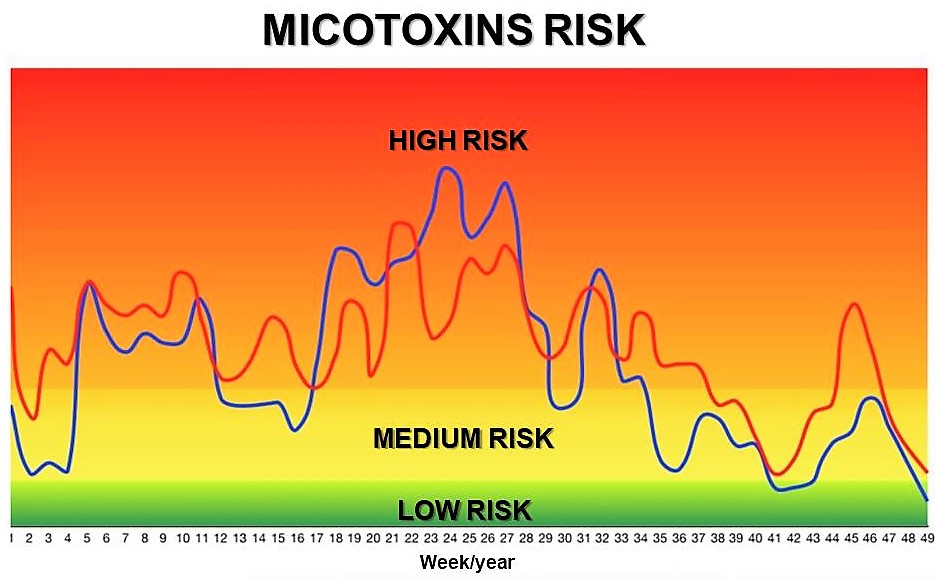
 3 - Results interpretation
3 - Results interpretation
The Mycotoxin Risk for each mycotoxin is calculated by Pegasus Science through the data which have been previously included by the Technician in charge. Calculation is carried out weekly and provides knowledge for the decision-making of offering (or not) a given raw material to a certain animal category, as well as about the need to include an Anti-mycotoxin Additive in the diet.
4 - Anti-mycotoxins Additives
Pegasus Science solely supports the use of Anti-mycotoxins Additives which follow the recommendations of the MAPA Workgroup on Mycotoxins. Criteria for in vitro and in vivo evaluations are established in these indications.





